The Challenge
Every year, 70,000 children in the United States are treated in emergency rooms for overmedication, according to the Centers for Disease Control and Prevention. A common cause of these life-threatening accidents: using ordinary spoons and cups to dose liquid medicines.
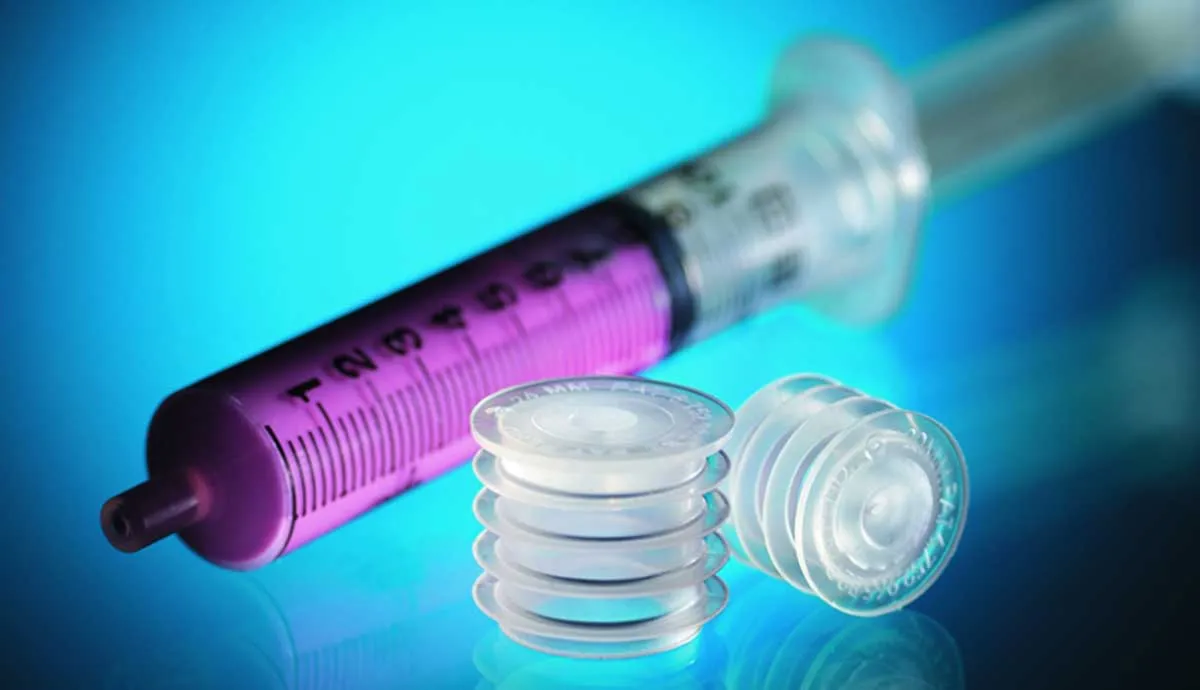
Product engineers at Andwin Scientific, a leading manufacturer of medical and laboratory supplies, were determined to find a replacement for these wildly imprecise measuring methods. Their efforts led to an ingenious solution: an adapter that fits snugly into the top of a medicine bottle, allowing a dosing syringe to be inserted.
The SealSafe® bottle dosing adapter lets consumers dose medicine the same way professionals do in a health-care setting – by inserting a syringe in the bottle, turning it over to eliminate air bubbles, and drawing a precise measure into the syringe. Once the syringe is removed, the adapter reseals and the cap can be put back on the bottle while the device remains in place.
At the heart of the concept is a patented self-sealing membrane. During development and prototyping, the engineers used an SBS-type elastomer for this critical component – but they hadn’t settled on materials for mass production.
Andwin Scientific sought out a supplier, knowing that any materials needed to be inert to liquid medicines such as amoxicillin – not to mention FDA-compliant.
The Solution
Seeking a partner that could provide consistency and local sourcing in North America and Asia, as well as establish drug master files for the United States and China, the Andwin Scientific team met with Avient, which has a range of technologies engineered to meet health care industry needs and regulations.
The two teams reviewed a range of possible materials for the dosing adapter, which consists of the self-sealing membrane and a cylindrical shell with flexible fins that lock the device in place. They ultimately selected a solution that utilized two custom formulations, both of which would meet FDA requirements.
The shell would be made with a custom-formulated Synprene™ thermoplastic elastomer (TPE) blend that provides resistance to most common medications and is flexible enough for the fins to work as designed. To assure the consistency of the product’s appearance and quality, the Synprene™ blend was designed to tolerate processing variability.
The membrane would be made from a custom-engineered GLS™ Dynaflex™ TPE overmolded onto the shell.
The Impact
Andwin Scientific’s patented solution improves dosing accuracy, allows the end-user to use every drop of medication and reduces the chance for an accidental spill. It also prevents air contamination and evaporation if the bottle isn’t closed right away. With the dosing adapter in place, medication bottles also have an additional layer of child-resistance.
When the product went into full production in the United States, Andwin Scientific realized a number of key gains:
- Better manufacturing efficiency: Cycle time for the two-part manufacturing process was 60% quicker than had been anticipated during development and production trials, and scrap rates were lower than expected;
- Lower costs: Annualized production costs came in at $30,000 less than budgeted.
Based on reduced lead times and supply chain costs to get the same formulations from Avient’s manufacturing facilities in China, Andwin Scientific is working to develop Asian markets for the SealSafe® adapter.
“Avient’s knowledge of materials, applied to our specific needs, gave us better consistency and quality,” says Andwin Scientific General Manager Ryan Smith. “Because Avient brought us a more consistent product, we have better process control and were able to reduce downtime and increase our production speed by 40 percent.”